|
Hexamethylenetetramine (HMT; chemical formula: C6H12N4, see Figure 1) was identified as a compound over 130 years ago. It was the first organic molecule on which X-ray crystallography was performed, and it was found to have tetrahedral symmetry. The infrared (IR) and Raman spectra of the solid and IR spectra of the gas have been reported, and the small number of observed fundamental vibration modes is in accord with the high degree of symmetry of this molecule (see references). The IR spectral properties and photochemistry of HMT and its variants, especially frozen in H2O-rich ices, are of astronomical interest, as they may comprise a non-negligible portion of interstellar ice grains, icy satellites, and the organic crust of comets.
Below is a representation of the HMT molecule in which the red spheres are nitrogen atoms, the black spheres are carbon atoms, and the white spheres are hydrogen atoms. Each nitrogen atom has three bonds to carbon, and each carbon has two bonds to nitrogen atoms and two bonds to hydrogen atoms.
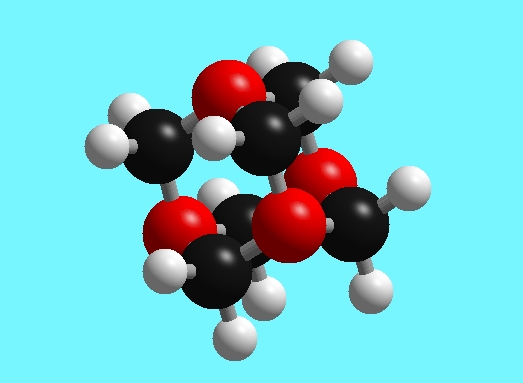
Figure 1: The structure of hexamethylenetetramine C6H12N4.
Red spheres are nitrogen atoms, the black spheres are carbon atoms, and the white spheres are hydrogen atoms.
HMT variants consist of HMT molecules in which one or more of the peripheral H atoms are replaced with chemical functional groups like –OH, –CH3, –NH2, –CH2OH, etc. Figure 2 shows examples of HMT variants in which one of the peripheral H atoms of HMT is replaced with various other functional groups.
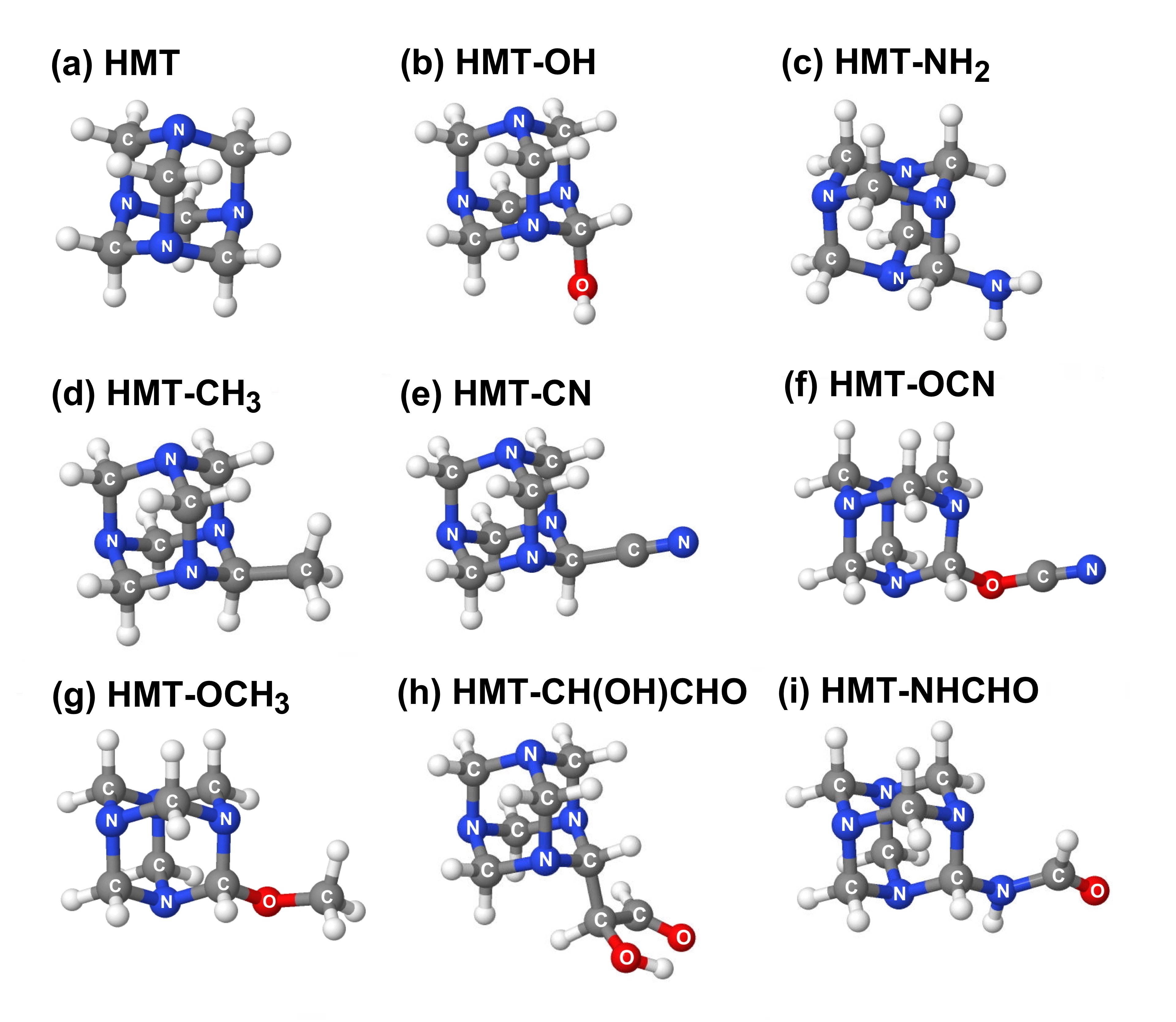
Figure 2: Examples of several variants of HMT-related compounds
which differ by the functional group attached to the HMT cage structure.
What do Hexamethylenetetramine (HMT) and HMT Variants have to do with the UV Photolysis of Interstellar, Protostellar, and Cometary Ices?
In our laboratory, we simulate the thermal and photochemical processes that occur in mixed molecular ices in physical environments like interstellar dense molecular clouds, protostellar disks, comets, and outer regions of our Solar System (for more details about these simulations, click here). Energetic processing of these ices has been shown to convert simple, abundant molecules (like H2O, CH3OH, NH3, CO, CO2, CH4, etc.) into more complex organic materials. The resulting populations of organics are very complex and include a number of classes of compounds that are of astrobiological interest, including amino acids, compounds related to polycyclic aromatic hydrocarbons (PAHs), amphiphiles, nucleobases, and sugar derivatives.
Among the most abundant products of ice irradiation is hexamethylenetetramine (HMT; C6H12N4) and a family of HMT variants. We have been able to identify HMT as a major component in our ice photolysis residues using a number of different techniques, including gas chromatography coupled to mass spectrometry (GC-MS), IR spectroscopy, and nuclear magnetic resonance (NMR) (see references). For example, the comparison of the IR spectrum of our organic residues to that of an HMT standard show that all of the peaks of HMT are seen in the spectrum of the organic material made in our laboratory simulations (Figure 3).
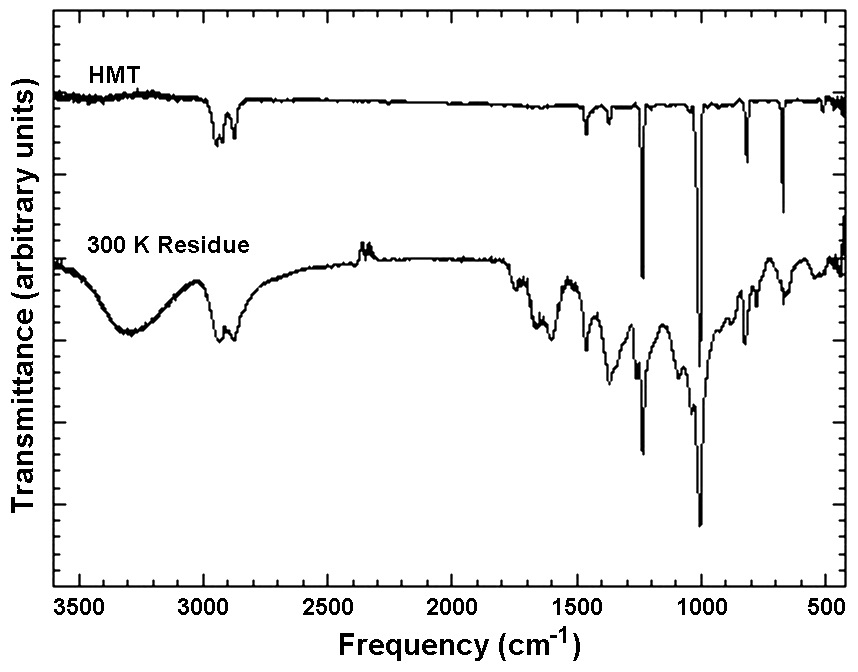
Figure 3: IR spectra of a sample of pure HMT frozen in H2O at 12 K
and subsequently warmed to 300 K (top trace) and of a residue produced from
the UV photolysis of an ice mixture containing H2O, CH3OH, CO, and NH3.
Using isotopic substitution techniques, we have also demonstrated that HMT-methanol is also produced in abundance in our ice irradiation experiments, and it is likely that a host of other HMT derivatives are also present
As a family of compounds, HMT and its variants constitute a major portion of the residues produced during ice irradiation, suggesting that, as a family, it may be possible to detect them in space using IR observations. We have calculated the IR spectra of many possible HMT variants (Figure 4). While each HMT variant produces a unique infrared spectrum, the spectra all share common spectral features associated with vibrations of the atoms in the central HMT skeletal structure and the overlap of these feature may result in strong enough features to be detected in space.
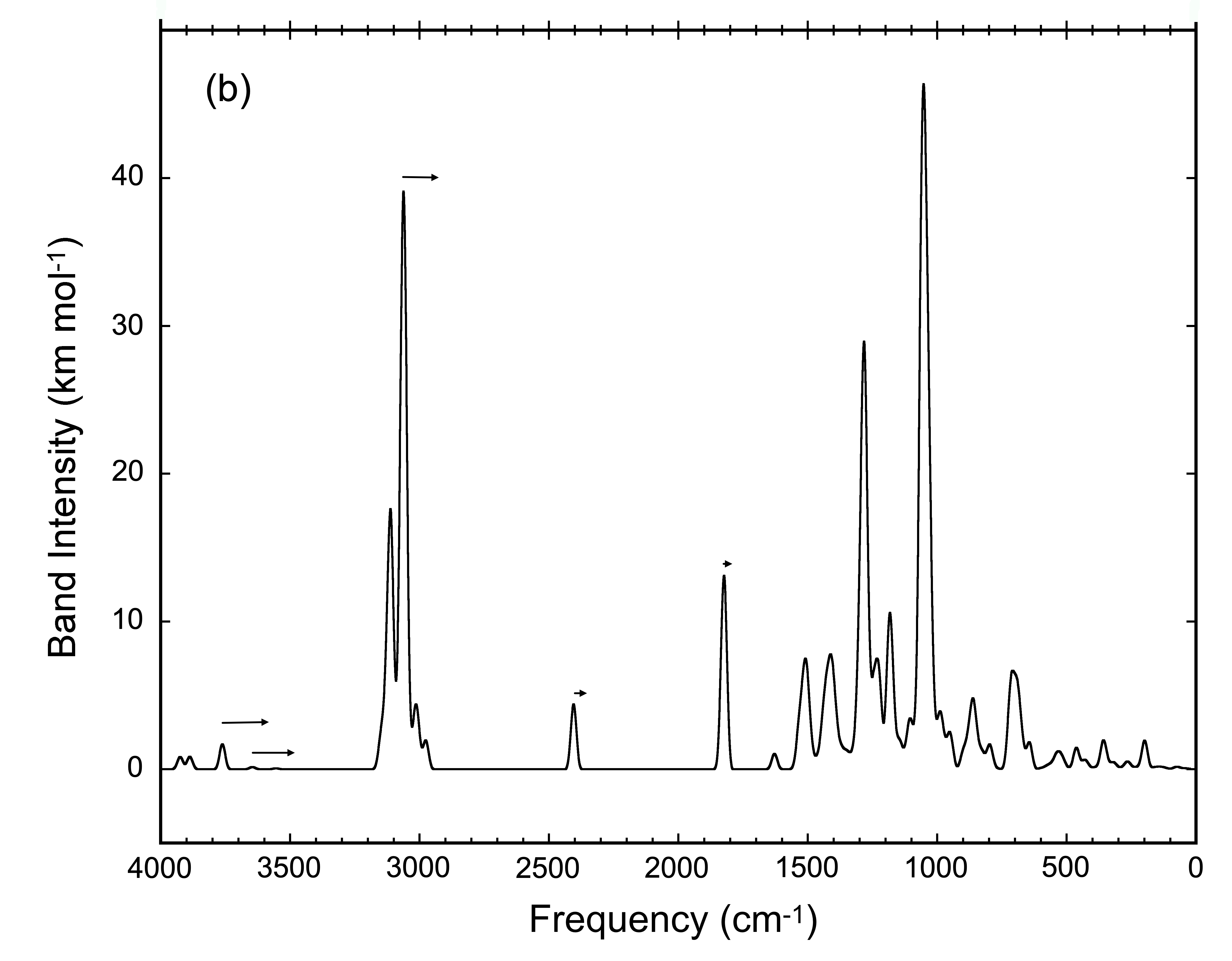
Figure 4: The composite spectrum produced by the overlapping
spectra of all the HMT variants shown in HMT Figure 2.
The exact mechanism by which HMT and its variants forms is not clear. However, it appears that the majority of these molecules are not made during the ice irradiation process, but rather when the irradiated ices are subsequently warmed. This suggests that HMT and its variants are made by the reaction of precursor molecules made during the irradiation phase. Key amongst these precursors is the molecule methyleneimine. Figure 5 shows a reaction path that may explain the mechanisms that lead to the production of HMT. The production of HMT variants may proceed in a similar manner in which one of the methylimines is replaced by a different reactant.
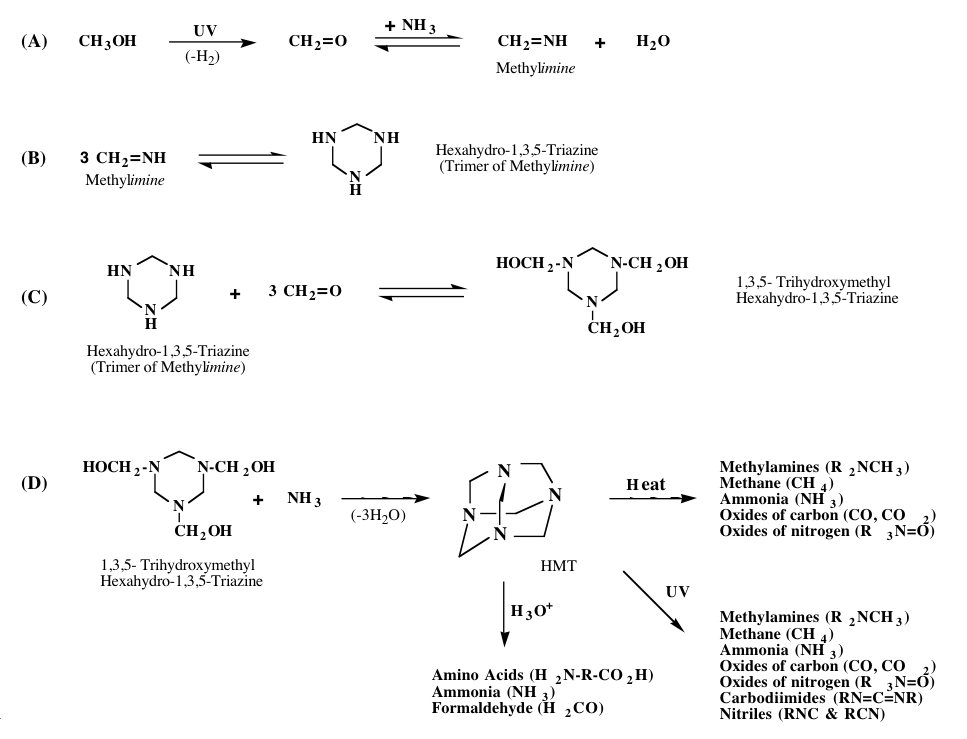
Figure 5: Proposed formation mechanism for HMT.
What is the Astrobiological Significance of HMT and its Variants?
This work suggests that HMT and similar molecules are likely to be present in non-negligible abundance in interstellar clouds, protostellar disks, comets, and other astrophysical environments in which ices are exposed to energetic radiation.
Once HMT and its variants are made, they can be further processed by exposure to heat, liquid water, or additional radiation (see Figure 5). When exposed to UV radiation or heat, HMT can break down to form a variety of new compounds, including cyanide compounds and oxides of carbon and nitrogen. Thus, HMT could be one of the parent molecules of CN seen in the comae of comets. The UV photolysis of HMT also produces “XCN,” a nitrile compound observed in cold astrophysical environments where CO and NH3 are present.
Of particular astrobiological interest is the observation that when this molecule is hydrolyzed in acidic solutions, it decomposes into a number of new compounds, including glycine and other amino acids. The hydrolysis of other HMT variants likely leads to the production of additional amino acids. Thus, HMT made in space and delivered to the early Earth may have played a role in terrestrial prebiotic chemistry and had implications for the origin of life.
For more detailed information and reviews on our laboratory work associated with HMT, see:
Materese, C. K., Nuevo, M., Sandford, S. A., Bera, P. P., & Lee, T. J. (2020). The Production and Potential Detection of Hexamethylenetetramine-Methanol and Other Hexamethylenetetramine Derivatives in Space. Astrobiology 20, 601–616.
Bera, P. P., Sandford, S. A., Lee, T. J., & Nuevo, M. (2019). The Calculated Infrared Spectra of Functionalized Hexamethylenetetramine (HMT) Molecules. Astrophys. J. 884, 64 (16 pp).
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Chang, S., & Scharberg, M. A. (1995). Organic Compounds Produced by Photolysis of Realistic Interstellar and Cometary Ice Analogs Containing Methanol. Astrophys. J. 454, 327–344.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., & Chang, S. (1994). Infrared Spectrum of Matrix-Isolated Hexamethylenetetramine in Ar and H2O at Cryogenic Temperatures. J. Phys. Chem. 98, 12206–12210.
Many of these can be downloaded from our Publications page.
|